how many electron does nitrogen have|Nitrogen (N) [7] — Chemical Element — Periodic Table : Pilipinas Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to . WEB1. IZA. A cantora IZA incendiou o clima na web ao postar cliques de um ensaio sensual em que surge nua no mar. Nas imagens, a artista aparece toda poderosa, esbanjando .
how many electron does nitrogen have,Element Nitrogen (N), Group 15, Atomic Number 7, p-block, Mass 14.007. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main content
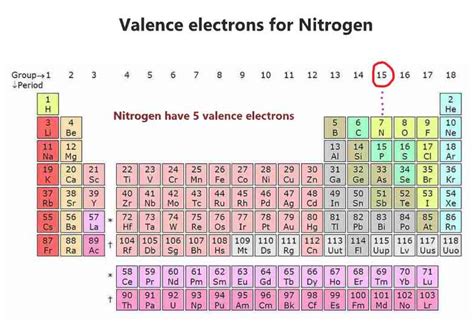
Members of a group typically have similar properties and electron configurations in .Nitrogen (N) [7] — Chemical Element — Periodic TableElectron affinity The energy released when an electron is added to the neutral atom . Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to .Nitrogen has seven protons and seven neutrons in its nucleus, and seven electrons in two shells. It is located in group fifteen, period two and block p of the periodic table. .A nitrogen atom has seven electrons. In the ground state, they are arranged in the electron configuration 1s 2s 2p x2p y2p z. It, therefore, has five valence electrons in the 2s and 2p orbitals, three of which (the p-electrons) are unpaired. It has one of the highest electronegativities among the elements (3.04 on the Pauling scale), exceeded only by chlorine (3.16), oxygen (3.44), and fluorine (3.9.
how many electron does nitrogen have Electrons have an electric charge of \(-1\), which is equal but opposite to the charge of a proton, which is \(+1\). All atoms have the same number of electrons as . Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom .Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only .
Nitrogen (N) [7] — Chemical Element — Periodic Table. ⬇. Physical data. Electronic data. Shells: 2, 5. Orbitals: [He] 2s 2 2p 3. Electronegativity: 3.0, 3.1. 1. Ionization potential: .
Nitrogen, nonmetallic element of Group 15 [Va] of the periodic table. It is a colorless, odorless, tasteless gas that is the most plentiful element in Earth’s atmosphere and is a constituent of all living .how many electron does nitrogen have Nitrogen (N) [7] — Chemical Element — Periodic Table Nitrogen has 5 electrons in its outermost orbit and it needs 3 more electrons to complete the octet configuration. Thus the nitrogen atom combines with the other nitrogen atom by sharing 3 .

The standard atomic mass of nitrogen is 14.006 and its symbol is ‘N’. The valence electrons are the total number of electrons in the last orbit (shell). The total number of electrons in the last shell after the electron configuration of nitrogen is called the valence electrons of nitrogen. The last shell of nitrogen has five electrons.

However, this is an incorrect perspective, as quantum mechanics demonstrates that electrons are more complicated. Figure 2.6.1 2.6. 1: Electrons are much smaller than protons or neutrons. If an electron was the mass of a penny, a proton or a neutron would have the mass of a large bowling ball!The simplest compound of nitrogen is molecular nitrogen, N2 N 2. The two nitrogen atoms are bonded together by a triple bond, consisting of a σ σ and two π π bonds. Molecular nitrogen, N2 N 2 [8] A common .
Nitrogen, nonmetallic element of Group 15 [Va] of the periodic table. It is a colorless, odorless, tasteless gas that is the most plentiful element in Earth’s atmosphere and is a constituent of all living matter. . electron configuration: 1s 2 2s 2 2p 3: History. About four-fifths of Earth’s atmosphere is nitrogen, which was isolated and .
Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s . This means to say that electron pairs only form after all orbital levels have been filled by unpaired electrons. For example, the ground state electron configuration of nitrogen ( 1 s 2 2 s 2 2 p 3 \rm 1s^22s^22p^3 1 s 2 2 s 2 2 p 3 ) indicates that it has 3 3 3 electrons occupying the 2 p 2 \rm p 2 p orbital.
How many core electron and valence electron does a nitrogen has? Nitrogen is the element located in group 15, period 2. Thus, its electron configuration is 1s2 2s2 2p3.
Nitrogen's atomic number is 7. That means that it has 7 positively charged protons in the nucleus. To be neutral, nitrogen must then also have 7 negatively charged electrons in its electron cloud.
how many electron does nitrogen have|Nitrogen (N) [7] — Chemical Element — Periodic Table
PH0 · Nitrogen (N) [7] — Chemical Element — Periodic Table
PH1 · Nitrogen (N)
PH2 · Nitrogen
PH3 · Electron Configuration for Nitrogen (N)
PH4 · Chemistry of Nitrogen (Z=7)
PH5 · 8.9.2: Chemistry of Nitrogen (Z=7)
PH6 · 2.6: Protons, Neutrons, and Electrons in Atoms